A Case of Fatal Drug Rash Eosinophilia and Systemic Symptoms from Allopurinol
- 1. Department of Biomedical Internal Medicine and Specialist, University of Palermo, Italy
- 2. Department of Sciences for the Promotion of Health and Mother and Child “Giuseppe D’Alessandro”, University of Palermo, Italy
Abstract
Hypereosinophilia is a systemic condition that has several possible etiologies: allergies, medications, infectious, autoimmune or other systemic diseases, and finally idiopathic forms. Skin involvement seems to relate to subcutaneous inflammatory infiltration in this condition, as can be observed in parasitic, autoimmune and bullous diseases, as well as in drug reactions. Generalizing, a severe adverse drug-induced reaction may cause a systemic inflammatory disease: Drug Rash with Eosinophilia and Systemic Symptoms (DRESS). Its diagnosis requires the application of a complex diagnostic algorithm and immediate identification to prevent inauspicious evolution. The prognosis is severe; drug discontinuation is sometimes not enough and so far the proposed therapies are not always valid. We describe a case of fatal DRESS in which we report: a) difficulties in the management and therapy of the syndrome in its most severe form, and b) need for caution in prescribing drugs potentially inducing DRESS, especially in elderly patients.
Citation
Maurizio S, Aurelio S, Giuseppe B, Giuseppe P, Maria FA, et al. (2015) A Case of Fatal Drug Rash Eosinophilia and Systemic Symptoms from Allopurinol. J Der matolog Clin Res 3(4): 1057.
BACKGROUND
Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome is a severe adverse drug-induced reaction (ADR) that occurs within 2-6 weeks after starting a pharmacological therapy [1]. This syndrome, also named DrugInduced Hypersensitivity Syndrome (DIHS), includes multiple systemic manifestations including an extensive cutaneous rash, fever, lymphadenopathy, and characteristic hematologic abnormalities such as eosinophilia, atypical lymphocytosis and rarely thrombocytopenia. Several organs, including liver, kidney, heart and pancreas, may be involved, individually or collectively, with eosinophil infiltration [1,2].
DRESS affects both adults and children, and although its precise incidence is unknown, the overall population risk has been estimated to range between 1:1000 and 1:10,000 drug exposures. DRESS is considered a life-threatening condition, with a mortality rate of about 10-20%, especially in the allopurinolrelated forms which are considered among the most severe and fatal [1-5]. Although DRESS was initially related to the use of anticonvulsants, any drug can cause the syndrome [1,2,6].
Several pathogenic hypotheses have been considered for DRESS:
1) Genetic susceptibility: the increased frequency of DRESS in some families and ethnic groups supports a role for possible genetic involvement. Descamps et al., hypothesized mutation in drug metabolism genes with accumulation of substances, which would trigger an autoimmune reaction and reactivation of some herpetic viruses [7]. Other authors suggest the existence of a predisposition linked to certain HLA variants; for example a relationship with HLA-B*5801 has been reported in the case of allopurinol [8-10].
2) Immune system disorders/herpetic viral reactivation: patients with DRESS display immune-suppression, probably induced by the drug, that would allow reactivation of some herpetic viruses such as HHV-6, HHV-7, CMV and EBV [1,2]. The latter condition is absent in the other toxidermia variants [11].
In particular, the identification of the HHV-6 genome is considered a diagnostic marker of DRESS [12]. Some authors believe that this is the first in (chronological order) of herpes viruses to reactivate; this could explain the clinical symptomology accounting for the similarities between DRESS and acute HHV-6 infection: visceral and cutaneous manifestations such as hepatitis, lymphadenitis, and pneumonia [1,2,7,13].
3) Type III hypersensitivity reaction: in the case of allopurinol it is assumed that one of its metabolites, oxopurinol, can lead to formation of immune complexes responsible for vasculitis through a type III hypersensitivity reaction. Moreover, oxopurinol is eliminated through the kidney, therefore people suffering from renal failure often develop allopurinol induced DRESS, probably due to greater drug accumulation [14,15].
Despite manifesting primarily as a dermatological pathology, the management of this syndrome in its more severe forms becomes multidisciplinary, owing to the systemic involvement and several iatrogenic complications; therefore, these patients are often admitted to internal medicine or intensive care units, and in such an environment it is necessary that DRESS be correctly recognized and treated. DRESS diagnosis is not always simple, and classically the three criteria proposed by Bocquet et al., [16] are used: 1) drug rash; 2) hematologic abnormalities (eosinophilia>1,500/mm3 and/or presence of atypical lymphocytes); 3) systemic involvement (adenopathy, diameter>2cm) and/or hepatitis (increase in transaminase of at least double the normal values), and/or interstitial nephritis and/or pneumonitis and/or carditis). Together with the one just mentioned, there are alternative criteria for the diagnosis of DRESS, including the Japanese study group of Severe Cutaneous Adverse Reactions to drugs (SCAR-J) [17], criteria adopted by the European group RegiSCAR [18], and finally the criteria proposed by Cacoub et al., [5] (Table 1).
|
Table 1: Scoring System for classifying DRESS cases from Cacoub et al. [5].
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the present report we describe a 78-year-old woman who developed an allopurinol-induced DRESS syndrome, together with a discussion of the key-points that challenged us in the diagnostic and therapeutic management of the condition.
CASE PRESENTATION
A 78-year-old Caucasian woman, with a history of hypertension, moderate chronic renal insufficiency (glomerular filtration rate 50mL/min) was admitted complaining of a weeklong history of weakness, fever >38°C, mental confusion and an extensive rash.
About three weeks prior to admission, the patient was started on allopurinol, 150mg/day per os, by her general practitioner for mild hyperuricemia, (7.5mg/dL, reference values 3.4-7.0mg/dL). After two weeks of treatment she complained of the sudden appearance of pruritus. Consequently, she stopped taking allopurinol and began symptomatic therapy with cetirizine, with itching disappearance in three days. When cetirizine was stopped pruritus reappeared immediately, and was accompanied by flu-like symptoms of fatigue, fever, and laterocervical lymphadenopathy, and the gradual development of an erythematous rash on the trunk and face. Due to the rapid worsening of the cutaneous rash and itching, as well as the persistence of other symptoms, she was admitted to our Department in stable clinical condition and a febrile. When admitted she was in stable clinical condition with a heart rate of 78 beats/minute and a blood pressure of 115/85mmHg. She was a febrile (T 36.8C), and eupneic with an oxygen saturation of 96%. Physical examination revealed a generalized erythematous maculopapular desquamative skin eruption, on the face, trunk and upper and lower limbs accompanied by pruritus (Figure 1).
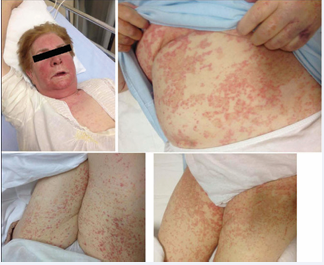
Figure 1 Patient, topographic distribution of the rash.
With the exception of submandibular and laterocervical .lymphadenopathy and fine crackles at the lung bases, no other significant physical findings were present. Laboratory investigations revealed leukocytosis with neutrophilia and eosinophilia, elevation of transaminases, lactic dehydrogenase (LDH), inflammatory markers (both C-reactive protein and erythrocyte sedimentation rate (ESR)) and kidney function markers, as well as hyperuricemia, hyperferritinemia, mild hyponatremia and hypokalemia (Table 2).
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The history of recent intake of allopurinol, followed by the appearance of systemic and cutaneous manifestations suggested the possibility of an ADR and, in particular due to the multiorgan involvement, DRESS syndrome. Other possible ADRs that should be considered in diagnosis are Stevens-Johnson syndrome and the acute generalized exanthematous pustulosis. However, several other diseases were considered in the differential diagnosis, particularly bacterial and viral infections, autoimmune (e.g. acute cutaneous lupus erythematosus) and neoplastic (e.g. angioimmunoblastic T cell lymphoma or Sézary syndrome) diseases, and the large spectrum of hypereosinophilic syndrome.
Blood and urine cultures as well as serologic determinations were obtained to exclude infectious diseases. These tests included serology for major and minor hepatitis-causing liver viruses (hepatitis A/B/C viruses (HAV, HBV, HCV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), as well as other important microbes causing hepatic infection, e.g. Salmonella spp, Brucella spp, Rickettsia spp, Chlamydia spp and Mycoplasma spp all of which were negative with the exception of a positive quantitative DNA response to Human Herpes Virus (HHV) 6. Diagnostic tests for autoimmune diseases, streptozyme, antistreptolysin titers and the most common autoantibodies, such as antinuclear antibody (ANA), extractable nuclear antigen (ENA), anti neutrophil cytoplasmic antibody (ANCA), antimitochondrial antibody (AMA) and anti-smooth muscle antibody (ASMA), were uniformly negative.
Abdominal and neck ultrasound (US), echocardiography and total body computerized tomography (CT) scans with intravenous contrast medium, showed no pathological abnormalities excluding a moderate volumetric increase of laterocervical lymphnodes. In view of the possibility of a hematological neoplasia e.g. lymphoid or myeloproliferative disorders, both morphological examination of peripheral blood smears and bone marrow aspirates were carried out and excluded hematological malignancies. However, these studies revealed abundant populations of eosinophil components accompanied by increased numbers of histiocytes showing prominent features of hemophagocytosis and intravascular hemopoiesis (Figure 2).
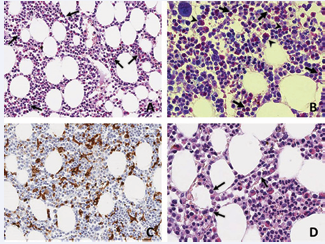
Figure 2 Bone marrow biopsy. A: The bone marrow parenchyma showed mild increase of hemopoietic cellularity and discrete degree of eosinophilia (arrows). Hematoxilyn eosin stain, x200. B: Giemsa staining showing the increase of the abundant mature (arrow) and immature (arrowhead) eosinophil granulocytic component. Giemsa stain, x400. C: Immunochemical staining with anti-CD68 highlights the increase of histiocytes with diffuse signs of hemophagocytosis. CD68 PG-M1, x200. D: Presence of myeloid precursors (arrows) within a dilated sinusoid (intravascular hemopoiesis). Hematoxilyn-eosin stain, x200.
The patient refused to undergo skin biopsy.
Collectively, these laboratory results coupled with the clinical findings of fever, systemic erythematous rash, hypertransaminasemia, leukocytosis, lymphadenopathy, and the recent ingestion of allopurinol supported the diagnosis of DRESS syndrome. The scoring system for classifying DRESS is outlined in the Table 1. Based on this scale, the patient had 8 of 9 criteria, which indicates a definite case of DRESS syndrome.
CLINICAL COURSE
Since high dose antihistamine therapy had been unsuccessful, prednisone 75mg/day per os was started during the first three days of hospitalization with initial clinical and laboratory benefit: fever disappeared, and cutaneous rash, lymphadenopathy and leukocytosis all attenuated (Figure 3).
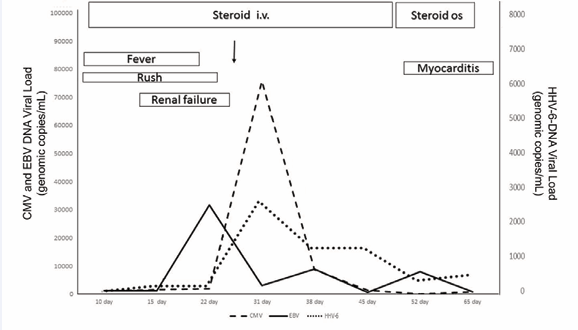
Figure 3 Clinical course of DRESS. Graphic description of the disease course, showing symptoms (fever, rash) and complications (renal failure and myocarditis), steroid treatment and serum viral load of cytomegalovirus (CMV), Epstein Barr virus (EBV) and Human Herpes Virus 6 (HHV6).
After five days of treatment, due to the sudden appearance of bronchospasm (characterized by wheezing with desaturation and dry sounds on physical examination, which resolved after administration of aerosolized bronchodilators and systemic steroids), and worsening of renal function (reduction of urinary output and increase of urea and creatinine serum levels associated with hyperkalemia and metabolic acidosis), the dose of prednisone was increased to 30mg/Kg daily, intravenously, for three days, with a beneficial effect in both renal function and respiratory parameters. Improvement in general condition, stabilization of kidney and respiratory functions and progressive disappearance of cutaneous erythematous lesions, which in the meantime attenuated and were replaced by fine desquamation of the superficial layers, led us to a gradual reduction in prednisone dose. Maintenance prednisone dose of 1mg/Kg/day i.v. was achieved on the 35th day after admittance. Such a long period of intravenous steroid treatment was necessitated by the frequent relapses every time we tried to switch to oral administration.
In the meantime, CMV and EBV DNA were measured weekly owing to the risk of viral reactivation, due to the high steroid doses and lymphocyte dysfunction typical of DRESS (Figure 3). On the 30th day a positive high viral load of both viruses was observed, reactivation was confirmed, and intravenous ganciclovir was initiated. On the 36th day, following the onset of a new febrile episode and increase in white blood cell count, blood culture was performed and was positive for methicillin-resistant Staphylococcus aureus; treatment with intravenous linezolid (600mg bid) was undertaken, followed by the disappearance of fever, and leukocyte reduction; echocardiography assessment excluded signs of endocarditis. Since the patient’s clinical condition remained stable, the dose of prednisone was reduced to 40 mg/day during the following days, and then switched to methylprednisolone (40mg/day) on the 48th day (Figure 3). Unfortunately, on the 52nd day, because of the onset of a condition compatible with acute myocarditis characterized by a supraventricular tachyarrhythmia with increase of the indices of myocardial necrosis and echocardiography showing systolic dysfunction, forced us to transfer the patient to cardiac intensive care (Figure 3). On this occasion it was not possible to perform MRI because the patient suffered from claustrophobia. In this context, a few days later a urinary infection caused by a multidrug-resistant Klebsiella pneumoniae, made its appearance and evolved into sepsis, leading to a further worsening of the clinical condition with septic shock and on the 70th day, the patient died.
CLINICAL COMMENTS
In the present case, a high index of clinical suspicion due to the recent allopurinol intake together with characteristic laboratory findings, e.g. eosinophilia, prompted the correct diagnosis. Laboratory and instrumental analysis excluded other possible causes such as infectious, immunologic or neoplastic diseases that must always be investigated. As already reported, to diagnose and classify our patient we used the criteria of Cacoub et al., [5], with a score of 8, therefore we classified her as: DRESS. In addition to the cutaneous involvement, mild hepatitis and mild nephritis were observed. Moreover, HHV-6 DNA positivity together with the above mentioned clinical, laboratory and instrumental data confirmed the hypothesis according to SCAR-J criteria also [17]. This did not allow us to perform skin biopsy, whose findings are considered of great importance by some authors [11]. The first measure we adopted was suspension of allopurinol, followed by steroid therapy on the third day. In cases like ours with moderate visceral involvement the Consensus of the French Society of Dermatology suggests prednisone 1mg/kg/ day. Using this dose, we succeeded in improving skin symptoms and hypereosinophilia, even though renal function worsened after 5 days of steroid therapy. For this reason we switched the steroid to methylprednisolone at a dose of 30mg/kg/day for three days as reported by some other authors [17]. Although the consensus of experts suggests the use of intravenous immunoglobulin in combination with steroid drugs in these cases, we did not use them because of severe renal impairment, and there is no agreement on their usefulness [19].
The high-dose intravenous steroid treatment was gradually reduced but when we tried the switch to oral therapy on the 40th day, we witnessed a revival of the skin symptoms. Nevertheless, we managed to improve the clinical condition even with oral steroid, until the resumption of the disease with myocarditis onset. As already mentioned, DRESS complexity lies not only in multiorgan involvement, but also in iatrogenic complications; during the course of the disease.Our patient contracted infection from multi-resistant bacteria, the first was a methicillin-resistant Staphylococcus Aureus treated according to susceptibility, and the second multi-resistant Klebsiella Pneumoniae, which was probably the cause of the exitus.
CONCLUSION
This case report leads to two observations: 1) Difficulties in the management and therapy of the syndrome in its most severe form. 2) Need to use caution with allopurinol in the treatment of asymptomatic hyperuricemia. 1) Difficulty in treatment: To date, although there are neither international guidelines nor randomized controlled studies for treatment of DRESS, a Consensus was established by the French Society of Dermatology that proposed a decisional tree of treatment based on the presence and severity of visceral manifestations [11]. One of the major limitations in DRESS treatment is the lack of reliable prognostic indicators as well as confirmed therapeutic protocols effective in the more severe forms; this is probably related to the rarity of the syndrome and the consequential impossibility of performing randomized controlled trials. Our case report expresses all these limits. The patient had severe evolution as evidenced by worsening during initial steroid treatment, and the rebound caused by switching to oral administration. In the initial stage many of the negative prognostic indicators reported in the literature were not present: eosinophils were <6.0 103/L and renal and hepatic involvement was mild. So there was no reason to expect a negative evolution or start a steroid treatment at higher dosage.
Another unclear aspect in severe forms is the duration of steroid therapy; although most studies agree on the rapid rebound of the disease on suspension of steroids [16,17] and propose a gradual reduction in 8 weeks, others recommend a duration of three months or more [1,2]. The treatment changes, however, are not well standardized, and there are no clinical or laboratory signs that could aid in switching from intravenous to oral therapy, or if the drug could be safely discontinued without a significant risk of relapse. The switch to oral therapy in our patient occurred when the clinical and laboratory signs normalized, but the disease relapsed unpredictably until the onset of myocarditis and the overlap of multi-resistant germ infections. It is unclear if our patient had a rare, but severe case of long-lasting steroid dependent variant of DRESS syndrome. The rapid progression with the overlap of a multi-resistant Klebsiella pneumoniae infection prevented us from using other therapy protocols.
2) Allopurinol use in asymptomatic hyperuricemia: our case raises questions about the use of allopurinol in patients with asymptomatic hyperuricemia. The use of the drug in gout has been well standardized for years, and recent guidelines have confirmed its usefulness, but do not offer clear indications as to its use in asymptomatic patients [20]. Several lines of evidence suggest treating hyperuricemia with drugs that reduce uric acid serum levels in the following conditions: 1) persistent hyperuricemia with serum urate concentrations above 13mg/dL in men and 10mg/dL in women; 2) uric acid urinary excretion greater than 1100mg daily in patients who do not respond to a purine-free diet; and 3) chemotherapy [6]. Recently, hyperuricemia treatment has received a great boost from the evidence from several studies that uric acid is a reliable marker of cardiovascular risk. Even in this context, however, a careful assessment considering the cost/benefit ratio in terms of cardiovascular risk/side effects is mandatory, especially in the elderly and patients suffering from renal failure. Recently, some authors have expressed doubts about the beneficial effects of the prevention of cardiovascular risk in patients with cardiorenal syndrome [21]. Finally, Stamp et al. proposed allopurinol doses adjusted to 1.5mg per 1 unit of the glomerular filtrate in patients with kidney failure, and possibly increasing progressively in relation to the urate serum levels; in our case, the patient required an initial dose of 75mg/dl [22].
Our case confirms that DRESS is an extremely severe adverse drug reaction characterized by high mortality rates. Currently the most severe forms remain difficult to treat and new approaches are desirable. However, in light of findings observed in our patient, it seems appropriate to carefully consider the usefulness of therapy with allopurinol in patients with serum uric acid levels that are only minimally elevated, especially in elderly patients and in other patients with reduced renal function.
CONSENT
Written informed consent was obtained from the patient’s daughter for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Maurizio Soresi: studied the case, reviewed literature and wrote the paper.
Aurelio Seidita: studied the case, reviewed literature and wrote the paper.
Giuseppe Brunori: studied the case and reviewed literature.
Giuseppe Pipitone: studied the case and reviewed literature.
Ada M Florena: performed pathological analysis and reviewed literature.
Salvatrice Mancuso: provided hematological consultation, performed pathological analysis and reviewed literature.
Anna Licata: studied the case and reviewed literature.
Gabriele Di Lorenzo: studied the case, reviewed literature and wrote the paper.
REFERENCES
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013; 68: 693.
- Criado PR, Criado RF, Avancini JM, Santi CG. Drug reaction with Eosinophilia and Systemic Symptoms (DRESS) / Drug-induced Hypersensitivity Syndrome (DIHS): a review of current concepts. An Bras Dermatol. 2012; 87: 435-449.
- Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken). 2013; 65: 578-584.
- Eshki M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009; 145: 67-72.
- Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011; 124: 588-597.
- Carnovale C, Venegoni M, Clementi E. Allopurinol overuse in asymptomatic hyperuricemia: a teachable moment. JAMA Intern Med. 2014; 174: 1031-1032.
- Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001; 137: 301-304.
- Dainichi T, Uchi H, Moroi Y, Furue M. Stevens-Johnson syndrome, drug-induced hypersensitivity syndrome and toxic epidermal necrolysis caused by allopurinol in patients with a common HLA allele: what causes the diversity? Dermatology. 2007; 215: 86-88.
- Cao ZH, Wei ZY, Zhu QY, Zhang JY, Yang L, Qin SY, et al. HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics. 2012; 13: 1193-1201.
- Gonçalo M, Coutinho I, Teixeira V, Gameiro AR, Brites MM, Nunes R, et al. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013; 169: 660-665.
- Descamps V, Ben Saïd B, Sassolas B, Truchetet F, Avenel-Audran M, Girardin P, et al. Eruptions Group of the French Society of Dermatology. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol 2010; 137: 703-708.
- Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007; 156: 1083-1084.
- Ichiche M, Kiesch N, De Bels D. DRESS syndrome associated with HHV-6 reactivation. Eur J Intern Med. 2003; 14: 498-500.
- Young JL Jr, Boswell RB, Nies AS. Severe allopurinol hypersensitivity. Association with thiazides and prior renal compromise. Arch Intern Med. 1974; 134: 553-558.
- Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984; 76: 47-56.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996; 15: 250-257.
- Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006; 55: 1-8.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007; 156: 609-611.
- Joly P, Janela B, Tetart F, Rogez S, Picard D, D'Incan M, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012; 148: 543-544.
- Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012; 64: 1431-1446.
- Patel KH, Goldsmith DJ. Uric acid as a cardiorenal risk factor - ready for prime-time? Int J Clin Pract. 2014; 68: 796-801.
- Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, et al.Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum. 2012; 64: 2529-2536.









































































































































